C9 -
Lipid droplet formation: Cooperative processes governing protein partitioning between membranes of distinct physicochemical properties
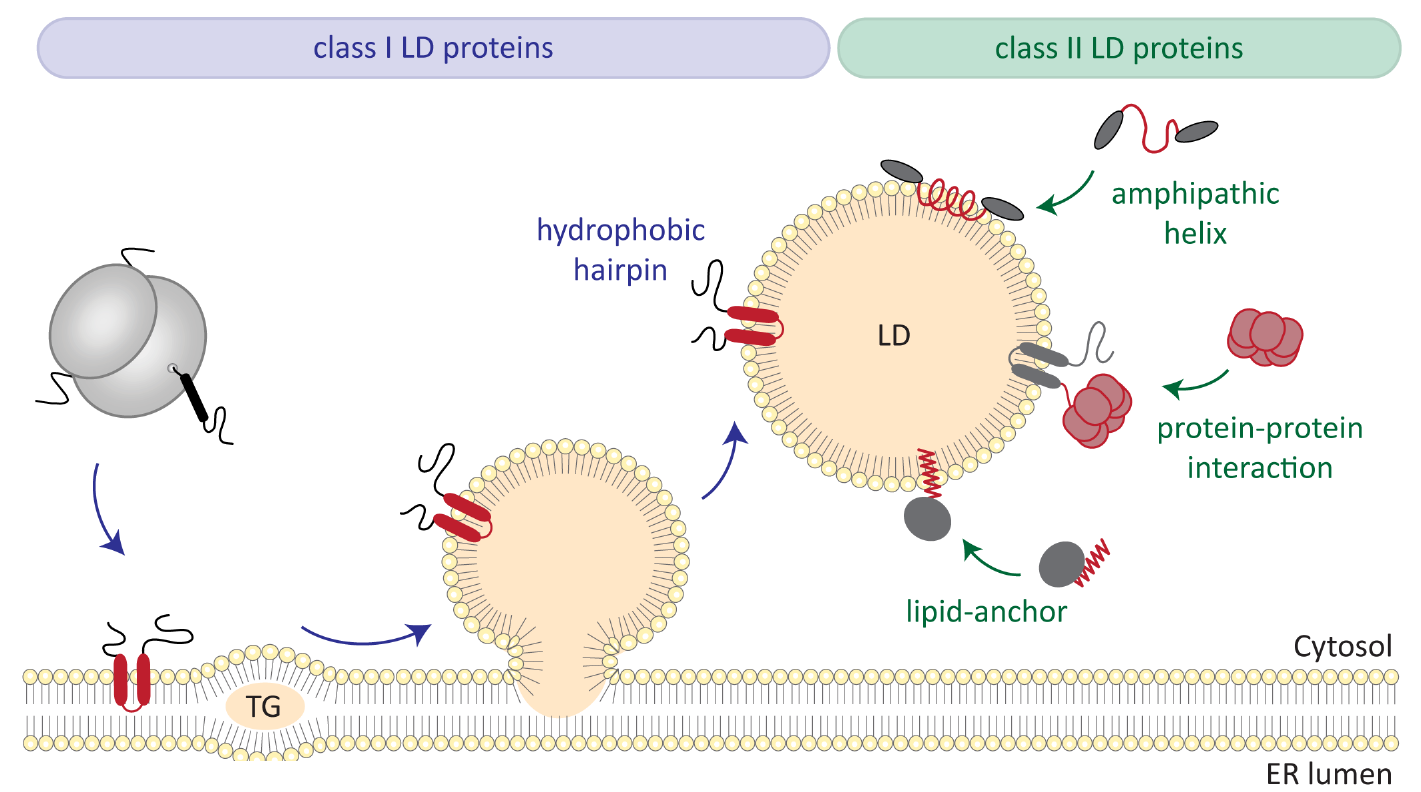
Lipid Droplets (LDs) are lipid storage organelles that create a unique physicochemical environment in the cell as their hydrophobic neutral lipid core is segregated from the aqueous cytosol by a phospholipid monolayer. The dynamic metabolic function of LDs relies on specific proteins that integrate into this membrane in a monotopic hairpin-type topology. This topology presumably facilitates partitioning of hairpin proteins from the endoplasmic reticulum bilayer membrane to LD monolayers; yet, the biophysical principles enabling hairpin proteins to reside in these distinct physicochemical membrane environments as well as the partitioning between them remain unknown. The central goal of this project is to determine which intrinsic protein features and which lipid-mediated parameters define a hairpin topology that allows bilayer-to-monolayer membrane partitioning. To this end a combination of biochemical and biophysical in vitro reconstitution experiments with molecular dynamics simulations is employed in a highly interdisciplinary approach.